Subjects
Grades
An exothermic reaction is a reaction that releases energy, thereby increasing the energy content of the surrounding medium.
When a chemical reaction releases heat into a medium, the temperature of that medium rises. The final temperature of the reaction is therefore higher than the initial temperature.
An exothermic reaction can be recognised in a number of ways :
An exothermic reaction is recognised when, in a chemical equation, the associated energy value (or thermal effect) is included on the product side of the reaction.
The equation for an exothermic reaction is of the type:
|\text{Reactants} \rightarrow \text{Products}+ \text{Energy}|
Methane combustion is an exothermic reaction:
|CH_{4(g)} + 2 O_{2(g)} \rightarrow CO_{2(g)} + 2 H_{2}O_{(l)} + 810 kJ|
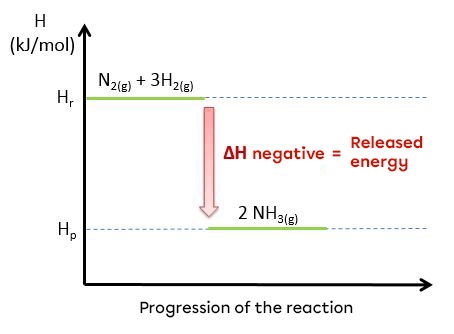
The synthesis of ammonia is an exothermic reaction:
|N_{2(g)} + 3 H_{2(g)} \rightarrow 2 NH_{3(g)} + 95,4 kJ|
The enthalpy (H) of a molecule is measured in joules per mole (J/mol ) or kilojoules per mole (kJ/mol). In an exothermic reaction, the enthalpy of the reactants is greater than that of the products. The change in enthalpy (ΔH) is therefore negative.
For an exothermic reaction
|H_{r} > H_{p}|
|\text{Reactants} \rightarrow \text{Products} \hspace {2 cm} \triangle H = \text {negative value}|
Here are two examples of exothermic reactions :
|CaO_{(s)} + CO_{2(g)} → CaCO_{3(s)} \hspace {2 cm} \triangle H = \text {- 178 kJ/mol}|
|N_{2(g)} + 3 H_{2(g)} → 2 NH_{3(g)} \hspace {2 cm} \triangle H = \text {-95.4 kJ/mol}|
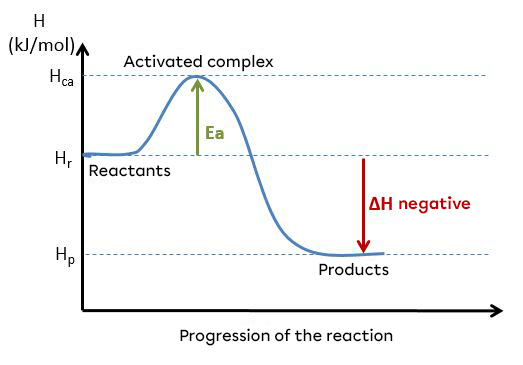
Here is a diagram showing an exothermic reaction and the change in energy during this reaction :
When bonds are formed in a molecule, energy is always released in the form of heat. This stage is always exothermic.
A reaction can be said to be exothermic when the direct activation energy (|E_{ad}|) is lower than the reverse activation energy (|E_{arev}|). These energies can be illustrated using an energy diagram.
For an exothermic reaction :
|E_{ad} < E_{arev}|
There are several examples of exothermic reactions in chemistry. The main ones are slow or rapid combustion and neutralisation reactions.
Example of an energy diagram for an exothermic reaction