Subjects
Grades
The rules of chemical notation are used to write the chemical formula (also called molecular formula) of a compound.
The following sections illustrate the notation rules that apply to binary compounds made of a metal and a nonmetal. A chemical bond between a metal and a nonmetal requires the formation of ions. Therefore, these sections focus specifically on binary ionic compounds.
To determine the chemical formula of a binary ionic compound, the charges carried by the ions that make up the compound have to be determined first.
The octet rule can be used to determine an ionic charge. It explains the element’s tendency to gain or lose electrons to form an ion.
The periodic table below shows the ionic charge(s) carried by an element in a binary ionic compound. This table applies to Elements 1 to 54.
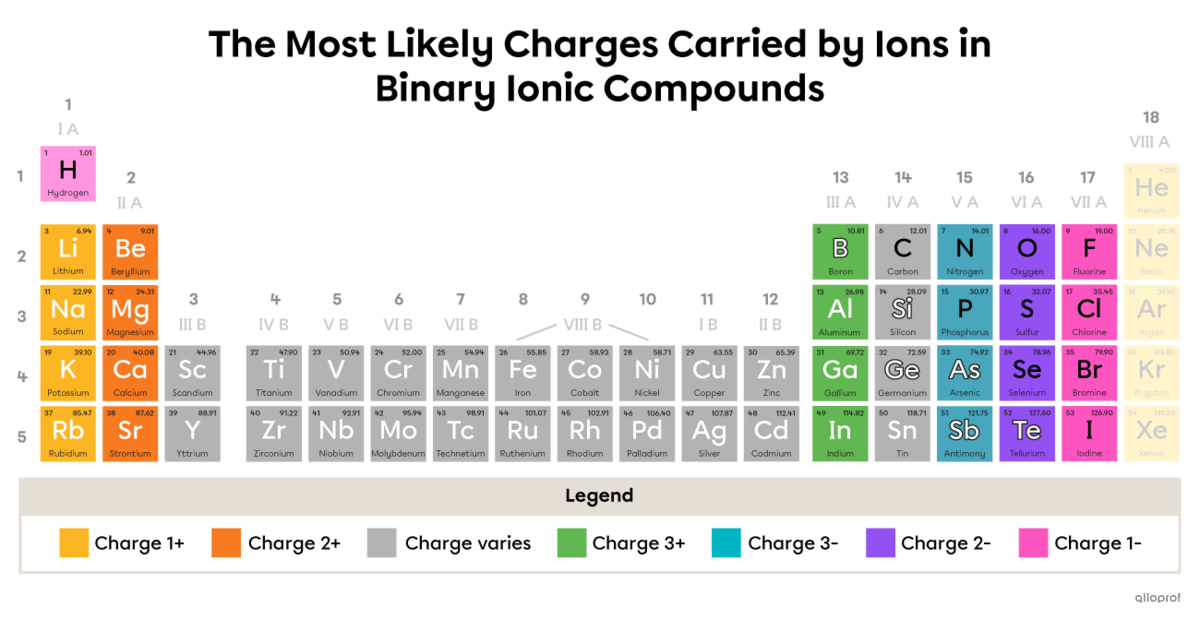
The charges indicated in the table represent the most likely charges carried by an ion in a binary ionic compound. Other charges are rare but possible.
In addition, the charges indicated in the table apply to binary ionic compounds. If a compound is ionic, but not binary, other ionic charges are possible. Ions are not involved in the formation of nonionic compounds.
In a binary ionic compound, what is the most likely charge of a magnesium ion?
In a binary ionic compound, what is the most likely charge of an aluminum ion?
In a binary ionic compound, what is the most likely charge of a copper ion?
To determine the chemical formula of a binary ionic compound based on its elements, the following rules of chemical notation should be used.
Determine the ionic charges carried by the elements that make up the compound.
Determine the number of ions of each element required to create an electrically neutral compound. The sum of all ionic charges must be equal to |0.|
Write the chemical formula with the metal first and the nonmetal last.
A compound is made of calcium |(\text{Ca})| and chlorine |(\text{Cl}).| What is the chemical formula of the compound?
A compound is made of lithium |(\text{Li})| and hydrogen |(\text{H}).| What is the chemical formula of the compound?
A compound is made of aluminum |(\text{Al})| and oxygen |(\text{O}).| What is the chemical formula of the compound?