Subjects
Grades
A fluid is a substance that can be deformed when subjected to a force. In other words, a fluid has the ability to take the shape of its container.
Fluids include gases, which are compressible fluids, and liquids, which are incompressible or are only slightly compressible.
The particles in gases and liquids are always in motion, continuously colliding with one another and the walls of the container.
These particles exert force on the surface of the container. This force, created by the number of collisions, is what generates pressure.

Moments in the video:
The volume of an incompressible fluid cannot, or can barely, be compressed into a smaller space. Liquids, such as water, oil and mercury, are incompressible fluids.
When the opening of a syringe is blocked by a finger and the plunger is pushed down to compress the water, the water will not compress: it will try to come out through the opening.
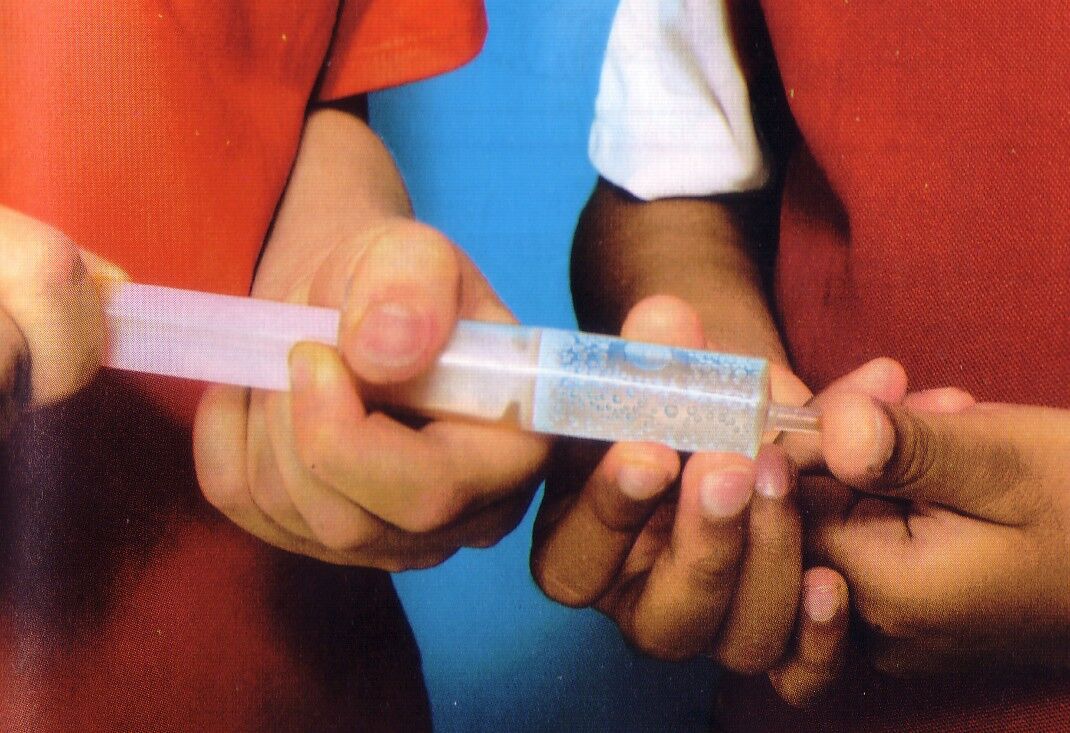
Liquid bodily fluids, such as blood and urine, are incompressible fluids.
In an incompressible fluid, there are two factors that affect pressure:
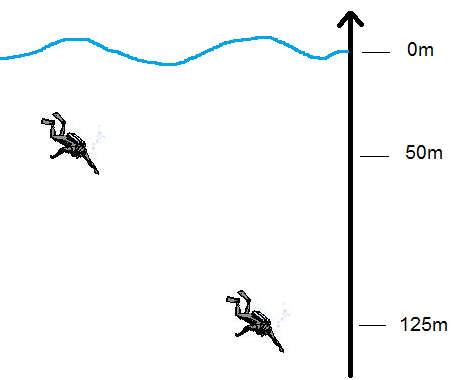
The pressure exerted on the object comes from the mass of the fluid above the object. The more liquid there is above the object, the greater the pressure.
The pressure exerted on a diver is greater at 125 m deep than at 50 m. This increase in pressure is noticeable in the lungs, because the diver is forced to use extra energy to inhale.
Density is another factor that affects the pressure of an incompressible fluid. The greater the density of the fluid, the greater the pressure exerted on the object placed inside it.
If two identical balloons filled with air are immersed in different fluids (water vs. oil) at the same depth, they are compressed differently because the two fluids have different densities.

Oil is less dense than water, so the balloon immersed in oil is less compressed than the one in water.
The volume of a compressible fluid can be changed and compressed into a smaller space by exerting pressure on it. All gases are compressible fluids (air, oxygen, hydrogen, nitrogen, etc.).
A fluid can be compressed if its particles are far from one another. When a force is applied to a gas, the distance between its particles is reduced. This reduces the space occupied by the gas.
Gaseous bodily fluids, like the air we breathe, are compressible fluids.
The pressure of a gas depends on the number of collisions that occur between the fluid’s particles and its container: more collisions result in higher pressure.
The following factors influence the number of collisions and, consequently, the pressure:
Number of particles in the fluid: If there are more particles, there are more collisions and a higher pressure.
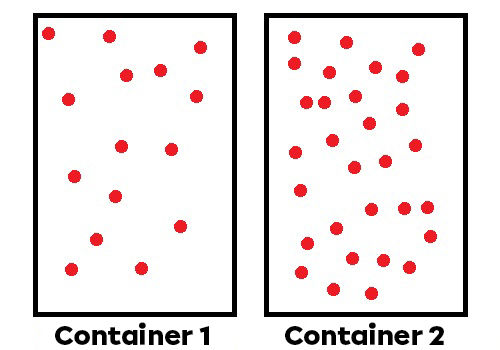
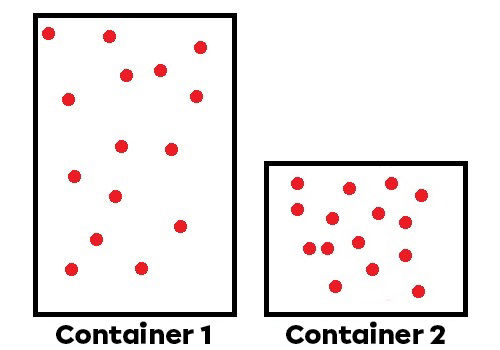
Volume of the fluid: If particles are contained into a smaller volume, there are more collisions and a higher pressure.
Temperature: Raising the temperature increases the energy of the particles, causing them to move faster and increasing the number of collisions and the pressure.
Because the body temperature of humans does not change very much, temperature is the only factor of the previous three that does not influence how a fluid moves. The circulation of fluids depends on the amount and volume of the fluid.
When a person inhales, the volume of the rib cage increases, and the pressure inside the lungs decreases. Since the atmospheric pressure is higher than the pressure inside the lungs, the outside air enters the lungs and equalizes the pressure.
The exhaling process is the opposite. The volume of the rib cage decreases causing an increase in pressure inside the lungs until it is higher than the atmospheric pressure. The air exits the lungs, equalizing the pressure.
Blood is an incompressible fluid, since its volume cannot decrease, but the pressure can change if the volume of the container is changed. When the heart contracts, the volume of the internal chambers of the heart decreases, and the pressure on the blood inside the heart increases. The blood is then expelled from the heart into the aorta or the pulmonary artery.
The opposite process is also true. When the heart relaxes, the volume of the heart increases, and the pressure inside the heart decreases. Blood entering the heart from the pulmonary veins, or vena cava, rebalances the pressure.