Subjects
Grades
Acidic substances and basic substances have characteristic properties that allow them to be differentiated. The table below provides a summary:
| Property | Acid | Base |
|---|---|---|
| Reaction with metals | Frequent reaction (usually gas production) | Little or no reaction |
| Electrical conductivity | Often high | Often high |
| Reaction of litmus paper | Turns blue litmus paper red | Turns red litmus paper blue |
| pH value | Less than 7 | Greater than 7 |
Acidity is the acidic character of a substance. It is a characteristic property of matter.
The acidity of a solution is rated using the pH scale.
Acidic substances have multiple properties. Here are a few:
Acidic foods have a sour taste (e.g., lemon, vinegar, etc.).
Some acidic solutions react with metals. This chemical reaction creates an effervescence due to the formation of hydrogen gas.
Acidic solutions conduct electricity. Acids are in fact electrolytes.
Acidic substances react with blue litmus paper. The paper turns red on contact with an acidic substance.
The pH of an acidic substance is less than 7.
The following images illustrate each of these properties:
Taste of food
Oranges are acidic and have a tart taste.

Reaction with metals
Iron reacts with an acid to generate a gas.

Litmus paper reaction
Acetic acid turns blue litmus paper red.

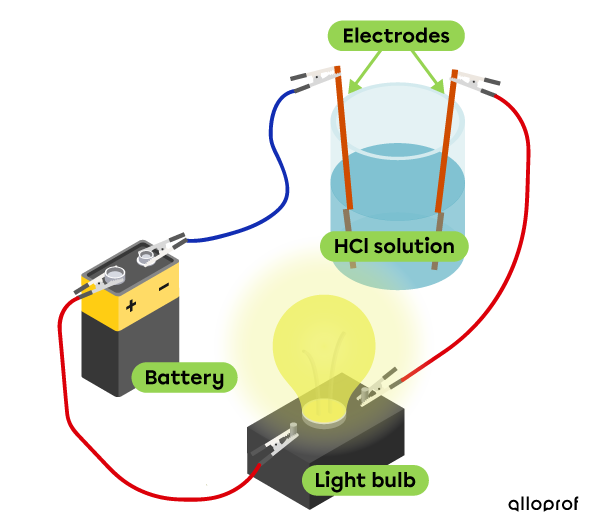
Electrical conductivity
Hydrochloric acid conducts electricity, which makes it possible for the bulb to be lit.
pH value
The pH meter indicates that this soil is slightly acidic (it has a pH of less than 7).

From a chemical point of view, there are several ways to define an acid. There are, in fact, several types of acids, and each has its own molecular formula.
Acids are present in food and are also used in certain industries, such as metallurgy, textiles, plastics, etc.
Apple vinegar contains acetic acid |(\text{CH}_3\text{COOH})|. It is often used to prepare marinades.

Apple vinegar
Citric acid|(\text{C}_6\text{H}_8\text{O}_7)|, found in lemons, is used as a preservative in food.

Lemons
Sulphuric acid|(\text{H}_2\text{SO}_4)| is used in automotive batteries.

A car battery
Aspirin is also an acid: acetylsalicylic acid|(\text{C}_9\text{H}_8\text{O}_4)|.

Aspirin
Basicity, also called alkalinity, is the basic character of a substance. It is a characteristic property of matter.
Basic substances have multiple characteristics. Here are a few:
Many basic solutions conduct electricity. In fact, bases are often good electrolytes.
Basic substances react with red litmus paper. The paper turns blue on contact with this base.
The pH of basic substances is greater than 7.
Unlike acids, the reaction of bases with metals is not particularly remarkable. Some bases react with metals, while others do not.
The following images illustrate some properties of the bases:
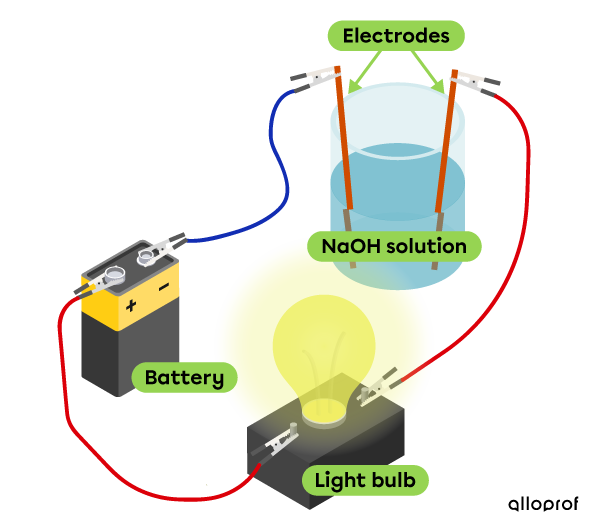
Electrical conductivity
This sodium hydroxide solution |(\text{NaOH})| conducts electricity, which makes it possible for the bulb to be lit.
Litmus paper reaction
Ammonia |(\text{NH}_4\text{OH})| turns red litmus paper blue.

pH value
The pH meter indicates that this solution is basic (it has a pH greater than 7).

From a chemical point of view, there are several ways to define a base. In fact, there are several types of bases, and each has its own molecular formula.
In general, bases are known to be effective in the composition of fertilizers and detergents. They are also used in metallurgy, the pulp and paper industry, food, pharmaceuticals, and in the plastics industry.
Ammonia |(\text{NH}_3)| is used in the production of margarine. In fact, ammonia is involved in the hydrogenation process, which consists of making sunflower oil into a solid.

Margarine
Calcium carbonate |(\text{CaCO}_3)| is used in the manufacture of antacids for heartburn.

Antacid tablets
Gels used to unclog pipes are very basic. They help dissolve hair and other material that clog the pipes. They also damage the skin, which is why wearing gloves is necessary.
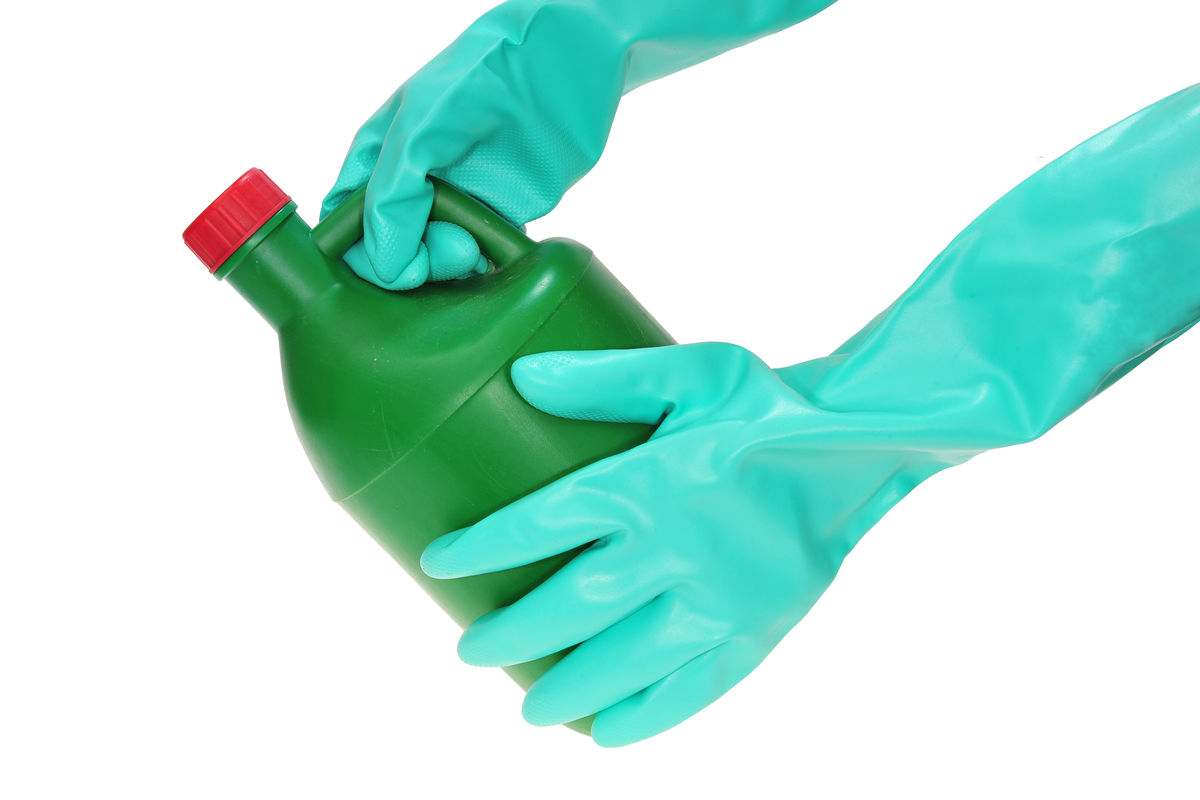
A gel is used to unclog pipes
Several techniques can be used to determine the acidity or basicity of a substance: litmus paper, pH paper, acid-base indicators, and the pH meter. The pH meter is the most precise of these methods, since it measures the pH of a solution to one or two decimal places.