Subjects
Grades
A chemical change, also known as a chemical transformation or chemical reaction, is a transformation of matter in which one or more substances interact to form new substances.
The substances found before and after a chemical change are different, meaning that they have different characteristic properties.
What's more, once the transformation is complete, it's not always possible to go back to the original substances. In this case, the transformation is said to be irreversible. Many chemical changes are irreversible.
Corrosion, cellular respiration, photosynthesis, combustion and decomposition are examples of chemical changes.
Corrosion is a chemical change in which a metal reacts with oxygen to form rust.

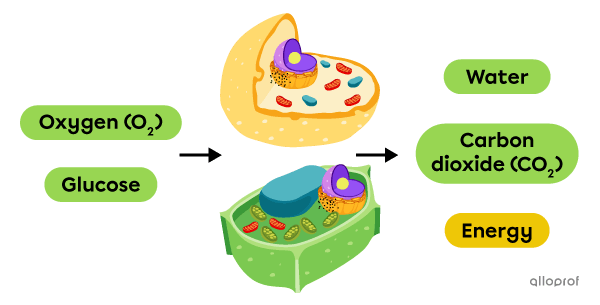
Cellular respiration is a chemical change that takes place in animal and plant cells. In this reaction, oxygen (O2) reacts with glucose, a sugar, to produce carbon dioxide (CO2) and water. This reaction also releases energy, which is then used by the cells.
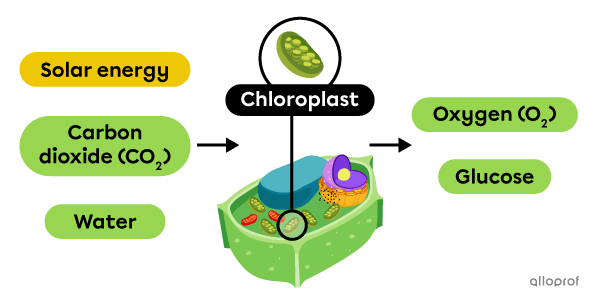
Photosynthesis is a chemical change that takes place in the chloroplasts of plant cells. By absorbing solar energy, plants convert carbon dioxide (CO2) and water into glucose and oxygen (O2).
Combustion is a chemical change. During the combustion of wood, it reacts with oxygen (O2) to form carbon dioxide (CO2) and water vapour. This reaction also releases energy in the form of heat and light.

The decomposition of certain foods during digestion is a chemical change.

Corrosion, cellular respiration and combustion are chemical changes in which an oxidation reaction takes place. During these changes, the original substances react with oxygen to produce new substances.
It's sometimes difficult to distinguish chemical and physical changes with the naked eye. However, the presence of at least one of the following clues generally points to a chemical change.
When two liquid substances come into contact, a chemical change sometimes occurs that produces a new solid substance. This substance is called a precipitate.
Cottage cheese can be made by mixing hot milk and white vinegar. When the two substances are mixed together, a white precipitate forms. The precipitate is cottage cheese.

When a potassium iodide solution is mixed with a lead nitrate solution, a yellow lead iodide precipitate is formed.

Certain chemical changes produce new gaseous substances. The release of gas can be noticed by the presence of bubbles in a liquid, also called effervescence, the presence of smoke or a characteristic odour.
When bread is made, the sugar is fermented by yeast, which results in a release of gaseous carbon dioxide (CO2). It is this off-gassing that causes the bread to puff up, sometimes leaving visible holes once the bread has finished baking.

When a tablet made of sodium bicarbonate and citric acid dissolves in water, it causes the formation of new substances, including carbon dioxide (CO2). This chemical change is accompanied by effervescence.

A colour change is a sign of chemical change, because it indicates the appearance of a new substance whose colour is different from the original substances.
The apple undergoes a chemical change when it comes in contact with oxygen in the air. It produces a new brownish substance.

When water is added to a coloured solution, a change in shade can be noticed. This change in shade should not be interpreted as an indication of chemical change. Rather, it is a physical change, called dilution, where no new substance is produced.

Some chemical changes absorb or release energy in the form of heat.
Food is cooked to become safe to eat or simply to change flavour or texture. Cooking food is a chemical change during which food absorbs heat.

Food undergoes a chemical change as it decomposes. A slight release of heat can be observed during the formation of compost.

Some chemical changes release energy in the form of light.
When a luminol-containing mixture comes into contact with iron, a new substance that emits blue light is formed.
Since blood contains iron, luminol is used during crime scene analysis to identify and analyze traces of blood invisible to the naked eye.

When a glow-in-the-dark bracelet is bent, certain substances inside come into contact and undergo a chemical change. One of the newly produced substances emits light.
