Subjects
Grades
Buoyancy is the ability of a substance to float or sink.
Buoyancy is due to several factors, including density. One substance floats on top of another substance if it is less dense.
So, for an object to float on water, it must be less dense than water. In other words, its density must be less than |1{.}0\ \text{g/mL}.|
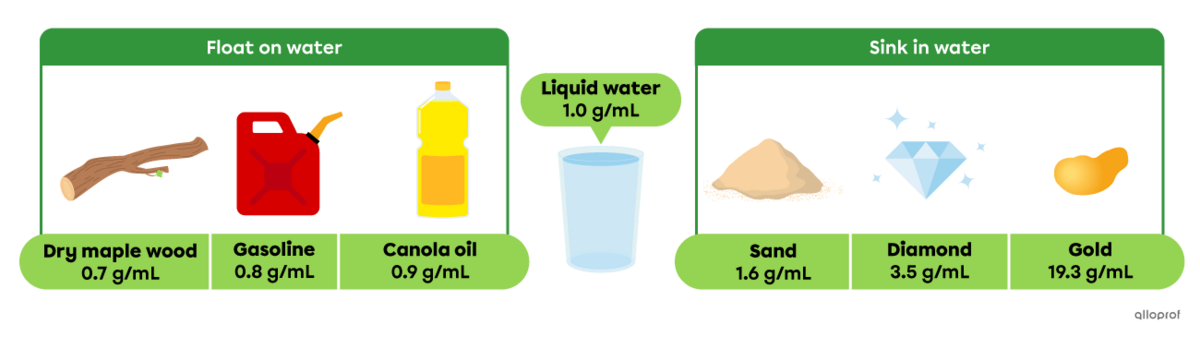
Dry maple wood, gasoline and canola oil float on water, since they all have a lower density than water (1.0 g/mL). Sand, diamonds and gold sink, since they all have a greater density than water.
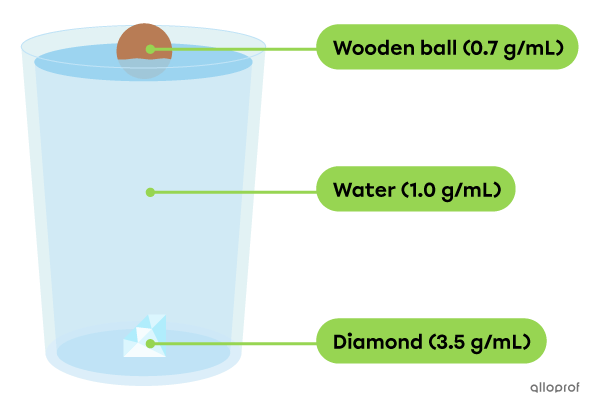
In a glass of water, you add a wooden ball and a diamond.
The wooden ball has a density of |0{.}7\ \text{g/mL}.| It's less dense than water, so it floats.
The diamond has a density of |3{.}5\ \text{g/mL}.| It is denser than water, so it sinks.
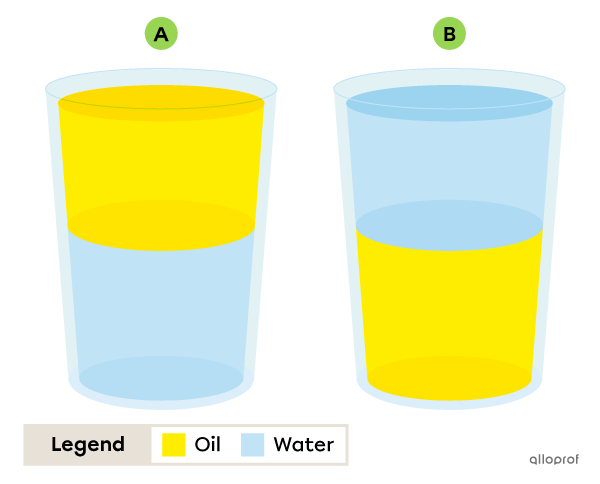
You mix water and canola oil in a glass. The density of the water is |1{.}0\ \text{g/mL},| while the density of the canola oil is |0{.}9\ \text{g/mL}.|
Which image corresponds to the resulting mixture?