Subjects
Grades
The water elctrolysis is a process that uses an electric current to separate the water molecule into its two elements.
The water molecule, |H_{2}O|, is a molecule formed by two hydrogen atoms and one oxygen atom. It is a pure substance that cannot be broken down by physical transformation. Instead, the molecule must undergo a chemical transformation in order to separate its different constituents.
In fact, a compound such as water, i.e. a molecule formed by the combination of two or more elements, can be broken down by chemical transformations. The process by which water is decomposed is called electrolysis. An electric current is passed through the water to produce two gases: hydrogen and oxygen. The following equation represents the decomposition of water:
|2 H_{2}O_{(l)} \rightarrow O_{2(g)}+ 2 H_{2(g)}|

The water electrolysis can be carried out experimentally. All you need is a current source, an acid solution and two electrodes, and you can set up the following diagram:
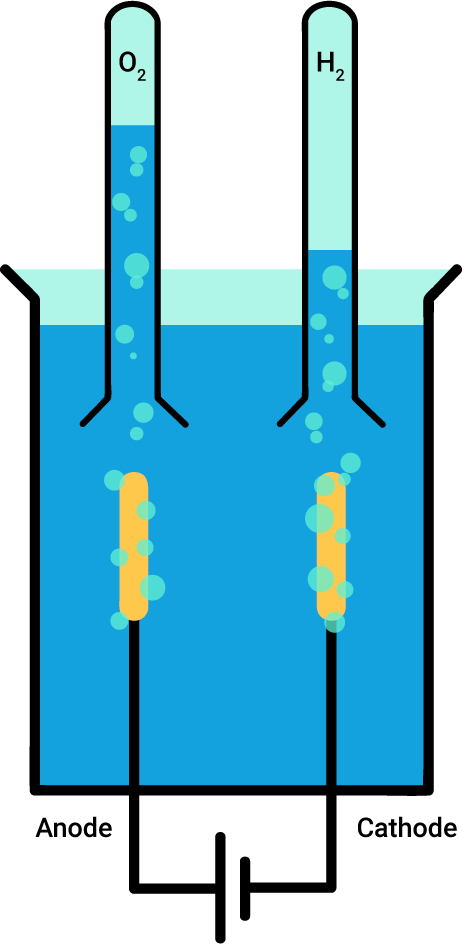
The experiment gives results that correspond to the chemical equation for water electrolysis: