Subjects
Grades
Choose your level.
A chemical reaction, sometimes called a chemical change or chemical transformation, occurs when one or more substances, the reactants, interact to form one or more new substances, the products.
A reactant is a substance that is used up during a chemical reaction. Its amount decreases.
A product is a substance formed during a chemical reaction. Its amount increases.
Unlike physical changes, a chemical reaction changes the nature of the substances involved. The reactants of a chemical reaction do not have the same characteristic properties as the products formed.
In addition, a chemical reaction can be recognized by certain indicators, such as a change in colour, the release of gas, the formation of a precipitate, etc.
Chemical reactions can be represented in different ways and grouped into categories referred to as the chemical reaction types.
Different representations can be used to describe the substances involved in a chemical reaction. A chemical reaction is usually represented using the particle model or a chemical equation.
When representing a chemical reaction using the particle model, atoms are usually represented by balls. The colour and size of the balls vary, allowing atoms of different elements to be represented differently. In addition, the balls can be combined to represent molecules and compounds.
The chemical reaction of carbon combustion can be represented using the particle model as follows. In this reaction, 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of carbon dioxide.
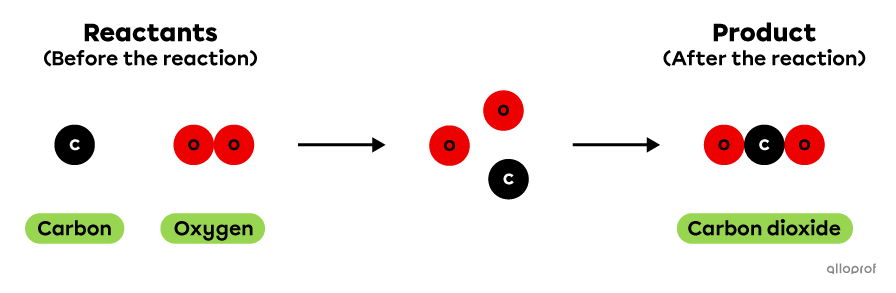
Carbon combustion according to the particle model
A chemical reaction can fit into several types of reactions at once. For example, cellular respiration is both an oxidation and a decomposition reaction.
A synthesis reaction is a chemical reaction where two or more reactants interact to form a new product.
A synthesis reaction can be recognized by analyzing the number of different reactants and products, as well as the complexity of the substances involved.
It is possible to recognize a synthesis reaction by the following clues:
There are more reactants than products.
The reactants are simpler substances than the product(s).
In the following reaction, ethylene reacts with hydrogen to form ethane.
This is a synthesis reaction because two reactants, ethylene and hydrogen, react to form one product, ethane. There are therefore more reactants than products. Furthermore, the two reactants are simpler molecules than the product: ethylene and hydrogen each contain fewer atoms than ethane.
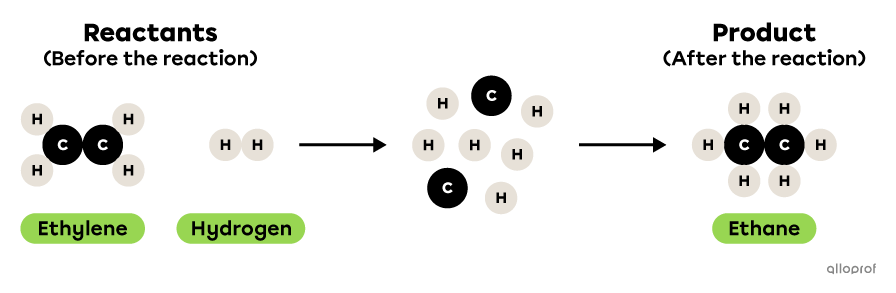
Ethane synthesis
Photosynthesis is a synthesis reaction where carbon dioxide reacts with water in the presence of solar energy to produce glucose and oxygen.
It can be recognized as a synthesis reaction because the glucose molecule produced is more complex than the two reactants involved.
In plants, this chemical reaction takes place in the chloroplasts.
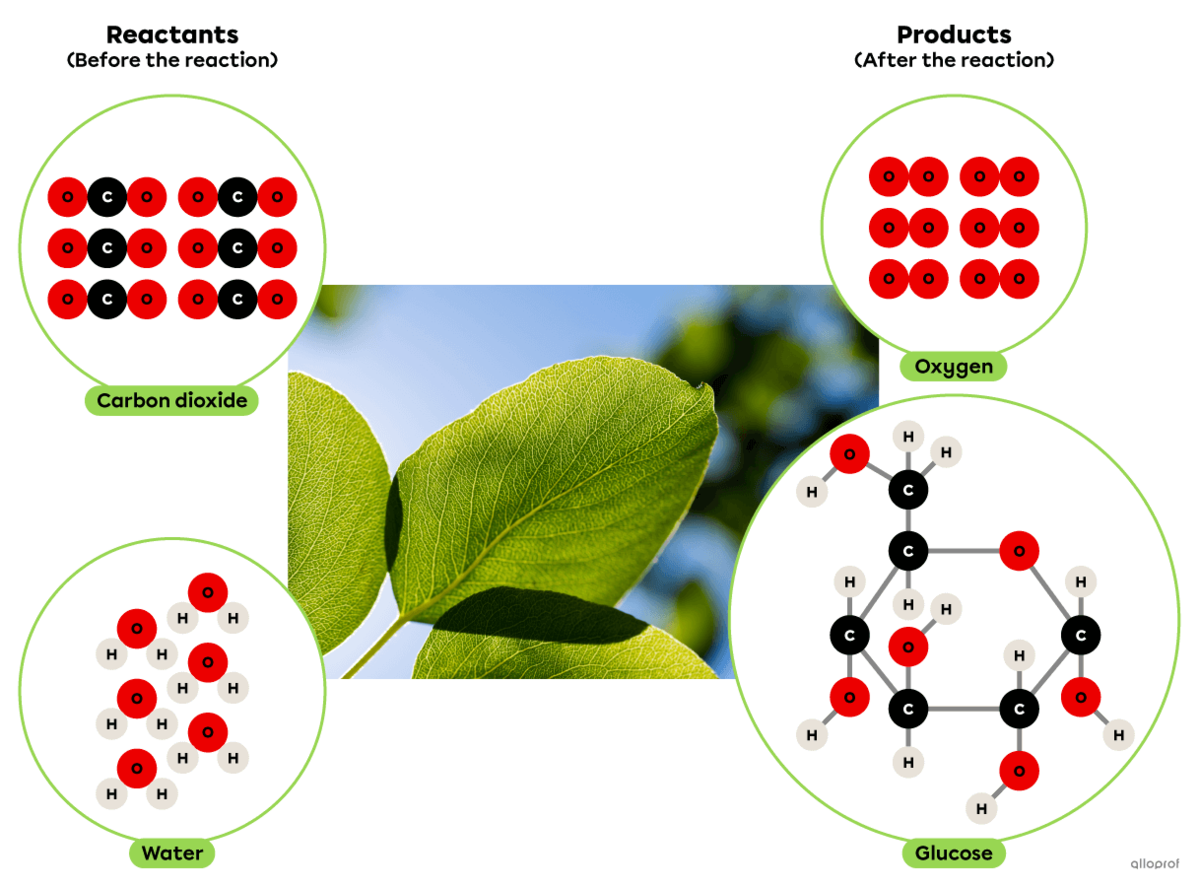
Photosynthesis according to the particle model
A decomposition reaction is a type of chemical reaction where the atoms of a reactant dissociate and rearrange to form several products.
A decomposition reaction can be recognized by analyzing the number of different reactants and products, as well as the complexity of the substances involved.
It is possible to recognize a decomposition reaction by the following clues:
There are more products than reactants.
The product(s) formed are simpler substances than the reactant(s).
In the following reaction, hydrogen peroxide decomposes to form hydrogen and oxygen.
It is recognized as a decomposition reaction because only one reactant, hydrogen peroxide, is decomposed to form two products, hydrogen and oxygen. In addition, hydrogen peroxide is a more complex molecule than either of the two products: it contains more atoms than either hydrogen or oxygen.
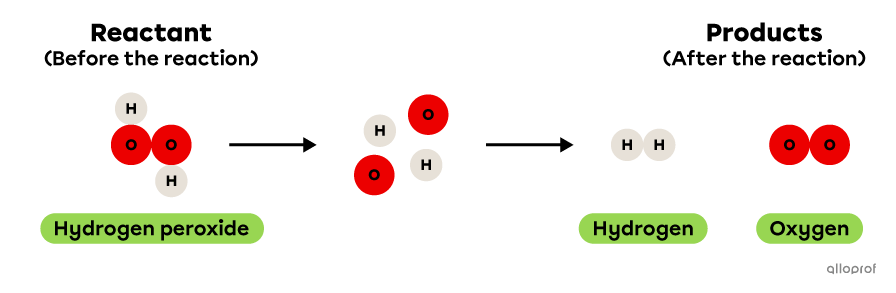
Decomposition of hydrogen peroxide
During chemical digestion, decomposition reactions allow nutrients, which are complex molecules, to be broken down into simpler molecules that can be absorbed by the body.
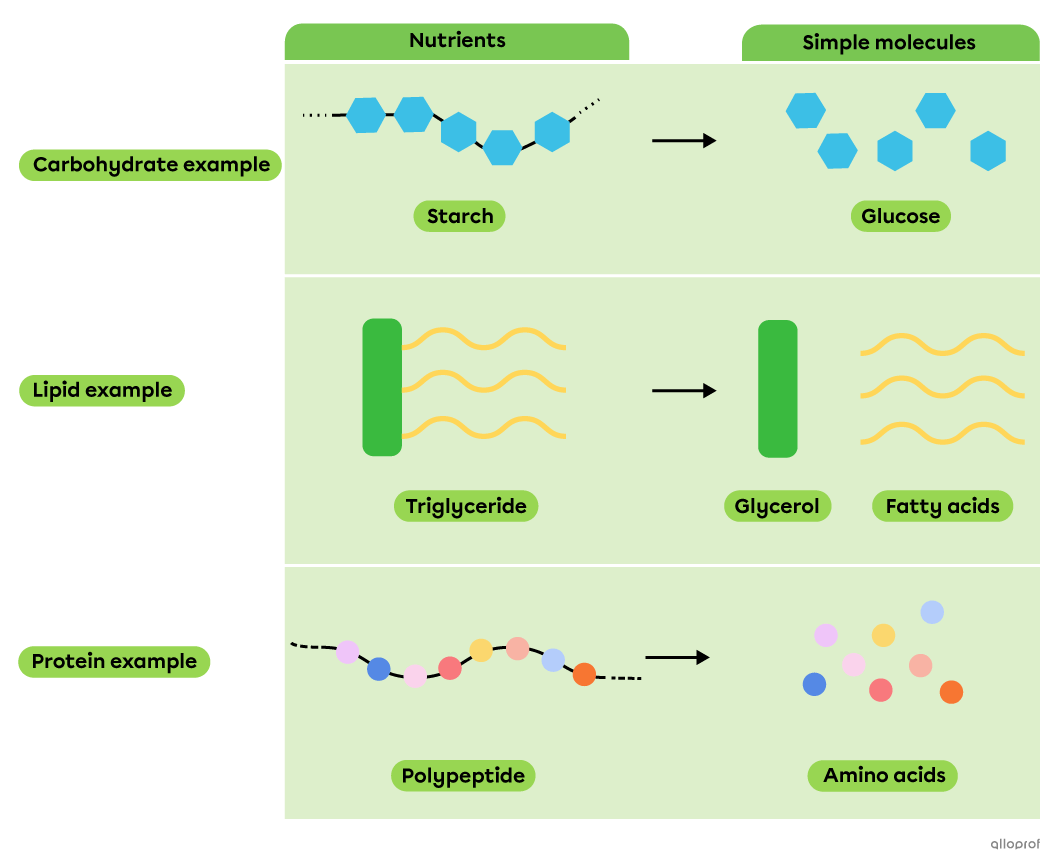
Decomposition of nutrients into simple molecules
A precipitation reaction is a chemical reaction that occurs when two substances in a solution interact to produce an insoluble or slightly soluble solid called a precipitate.
When a sodium chloride solution is mixed with a silver nitrate solution, a whitish precipitate is formed.
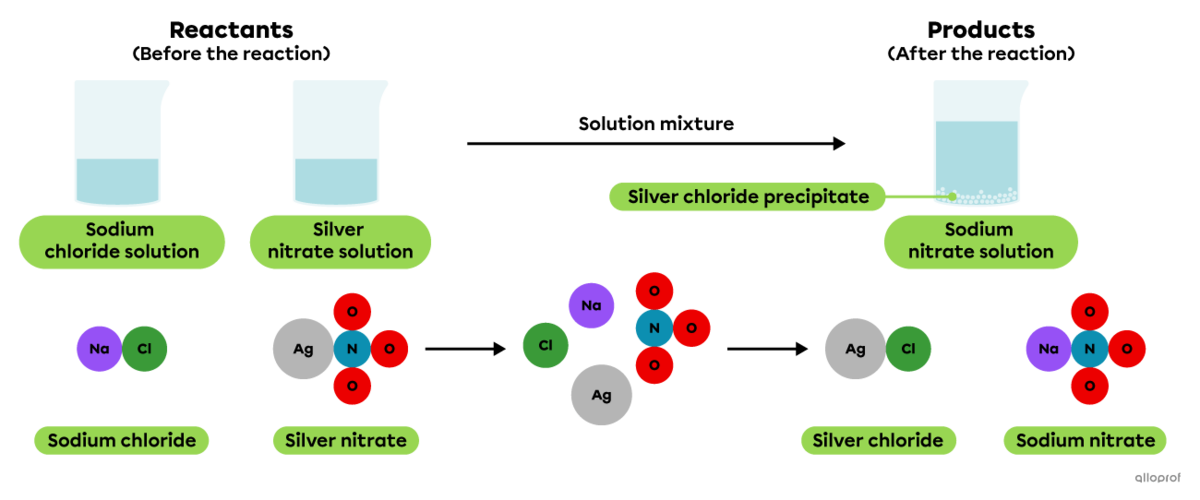
Precipitation of silver chloride
An oxidation reaction is a chemical reaction involving oxygen or a substance with oxygen-like chemical properties among the reactants.
In the following reaction, iron reacts with oxygen to form iron oxide, commonly known as rust. This is an oxidation reaction because oxygen is one of the reactants involved in the reaction.
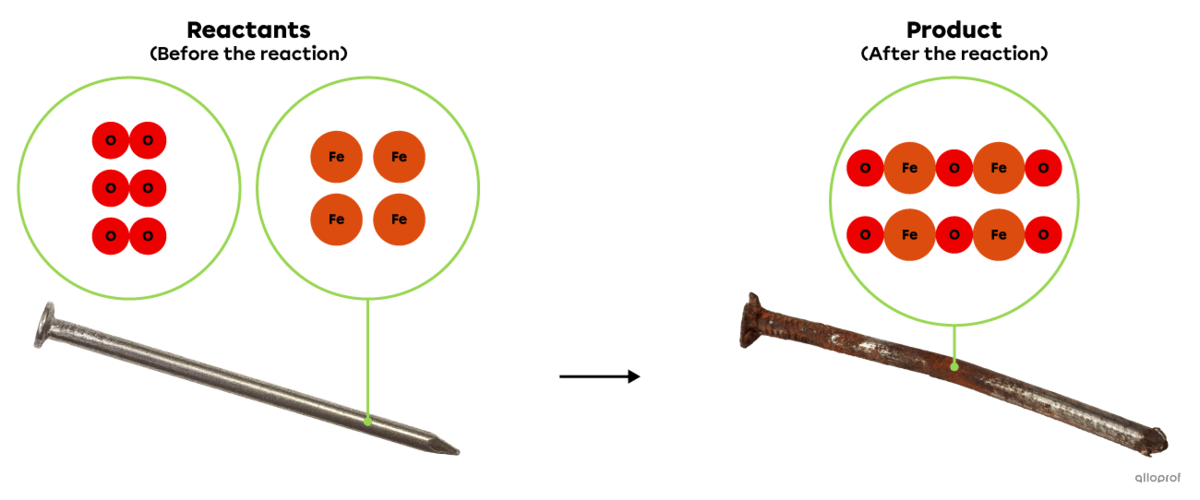
Oxidation of iron
A combustion reaction is a chemical reaction where an oxidation reaction occurs and energy is released.
In the following combustion reaction, propane reacts with oxygen to form carbon dioxide and water. In addition, the combustion of propane results in the release of thermal energy which is commonly used for cooking or heating buildings.
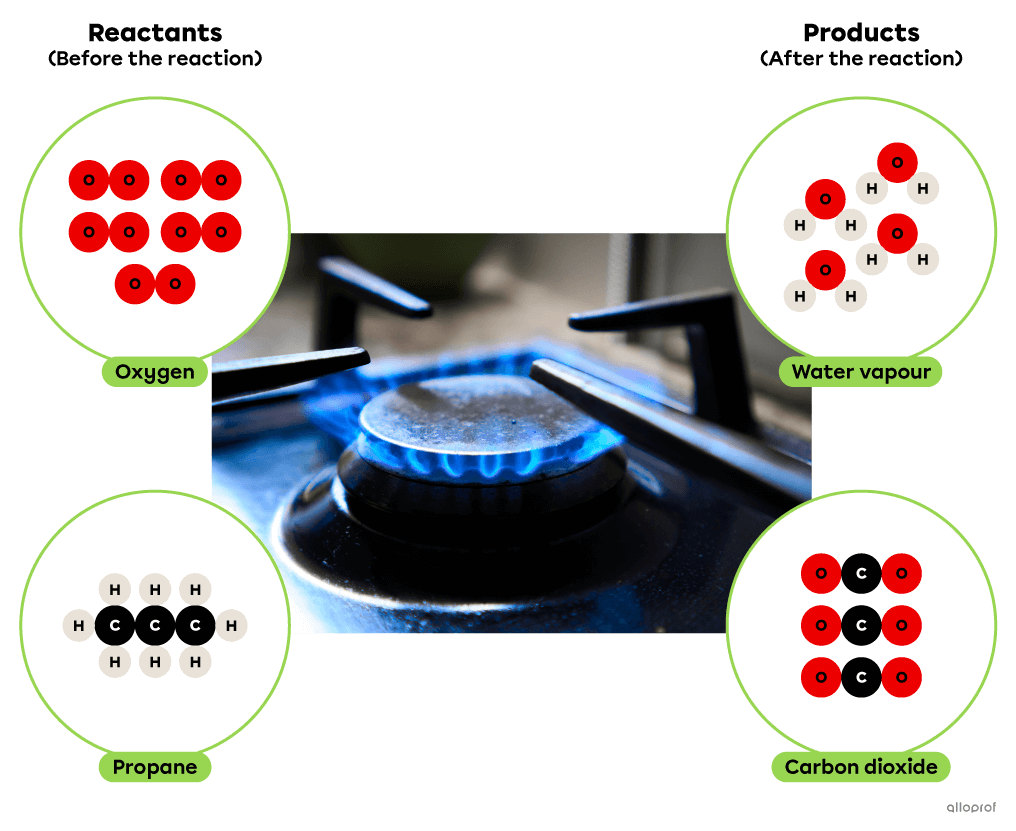
Combustion of propane
Cellular respiration is a chemical reaction that takes place in animal and plant cells.
It is an oxidation reaction where glucose reacts with oxygen to form carbon dioxide and water vapour. In addition, cellular respiration produces the energy essential for the proper functioning of cells.
Since it involves an oxidation reaction and the release of energy, it can also be said that cellular respiration is a combustion reaction.
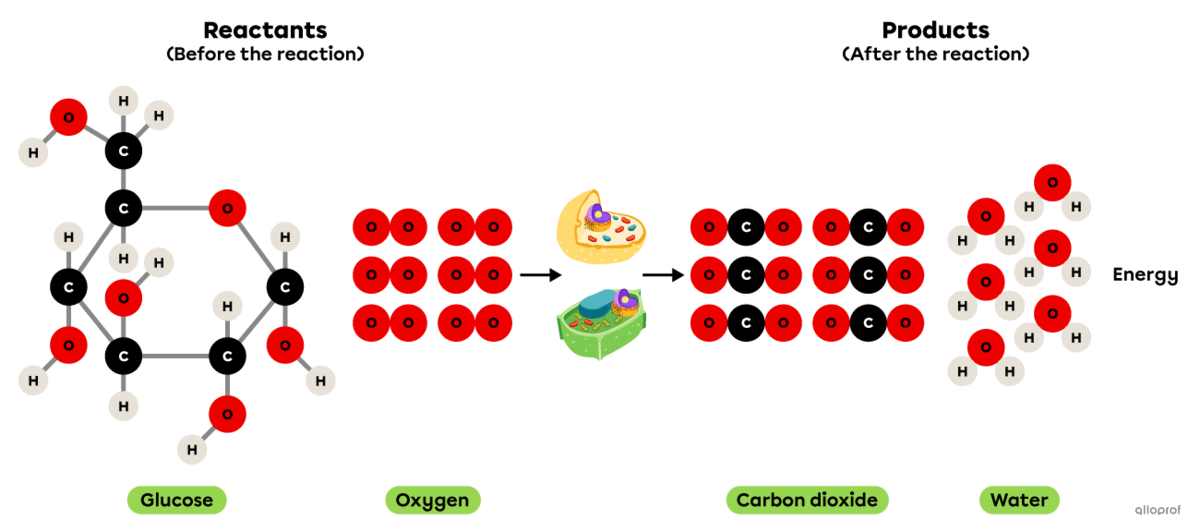
Cellular respiration
A chemical reaction, sometimes called a chemical change or chemical transformation, occurs when one or more substances, the reactants, interact to form one or more new substances, the products.
A reactant is a substance that is used up during a chemical reaction. Its amount decreases.
A product is a substance formed during a chemical reaction. Its amount increases.
During a chemical reaction or a chemical change, the chemical bonds between the atoms of each reactant are broken. As a result, the atoms rearrange themselves to form new bonds, enabling the formation of products.
Unlike physical changes, a chemical reaction changes the nature of the substances involved. The reactants of a chemical reaction do not have the same characteristic properties as the products formed.
In addition, a chemical reaction can be recognized by certain indicators, such as a change in colour, the release of gas, the formation of a precipitate, etc.
Chemical reactions can be represented in different ways and grouped into categories referred to as the chemical reaction types.
Different representations can be used to describe the substances involved in a chemical reaction. A chemical reaction is usually represented using the particle model or a chemical equation.
When representing a chemical reaction using the particle model, atoms are usually represented by balls. The colour and size of the balls vary, allowing atoms of different elements to be represented differently. In addition, the balls can be combined to represent molecules and compounds.
The chemical reaction of carbon combustion can be represented using the particle model as follows. In this reaction, 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of carbon dioxide.

Carbon combustion according to the particle model
A chemical equation is a schematic representation of the substances involved in a chemical reaction written as an equation.
In a chemical equation:
Reactants and products are represented by their chemical formulas.
Reactants and products are separated by an arrow.
If a chemical reaction involves more than one reactant or product, their chemical formulas are separated by a plus sign |(+).|
Coefficients may be placed in front of the chemical formulas of reactants and products to represent the actual proportions in which the substances interact.
Therefore, a chemical equation usually looks like this:
||\begin{align}\color{#FA7921}{\text{Reactants}}&\rightarrow\color{#333FB1}{\text{Products}}\\\color{#3A9A38}{a}\color{#FA7921}{\text{A}}\ +\ \color{#3A9A38}{b}\color{#FA7921}{\text{B}}&\rightarrow\color{#3A9A38}{c}\color{#333FB1}{\text{C}}\ +\ \color{#3A9A38}{d}\color{#333FB1}{\text{D}}\end{align}||
where
|\color{#FA7921}{\text{A, B}}:| Chemical formulas of reactants
|\color{#333FB1}{\text{C, D}}:| Chemical formulas of products
|\color{#3A9A38}{a,b,c,d}:| Stoichiometric coefficients
In the majority of situations encountered in high school, a one-way arrow pointing towards the products is used to separate the reactants from the products. This arrow is used when the reaction is irreversible, meaning that one of the reactants is completely used up and transformed into products.
||\text{Reactants}\rightarrow\text{Products}||
Sometimes the products formed during a chemical reaction react to generate reactants again. These reactions are called reversible and a double arrow is used to separate the reactants from the products.
||\text{Reactants}\leftrightharpoons\text{Products}||
A skeleton equation is a chemical equation used to represent the substances involved in a chemical reaction without indicating the actual proportions in which the substances interact.
In a skeleton equation, the chemical formulas of the substances involved are found without the stoichiometric coefficients in front of them. In other words, a skeleton equation is an unbalanced chemical equation.
The combustion reaction of starch can be represented by the following skeleton equation.
||\color{#FA7921}{\text{C}_6\text{H}_{10}\text{O}_5}\ +\ \color{#FA7921}{\text{O}_2}\rightarrow\color{#333FB1}{\text{CO}_2}\ +\ \color{#333FB1}{\text{H}_2\text{O}}||
According to this equation, starch |(\color{#FA7921}{\text{C}_6\text{H}_{10}\text{O}_5})| reacts with oxygen |(\color{#FA7921}{\text{O}_2})| to form carbon dioxide |(\color{#333FB1}{\text{CO}_2})| and water |(\color{#333FB1}{\text{H}_2\text{O}}).|
A balanced chemical equation is a type of chemical equation that indicates the actual proportions in which substances interact during a chemical reaction.
To represent the actual proportions in which substances interact during a chemical reaction, the equation must reflect the law of conservation of matter and have an equal number of atoms of each element on both sides of the equation (on the reactant and product sides).
To achieve this, the chemical equation must be balanced. Balancing chemical equations is done by placing stoichiometric coefficients in front of the chemical formulas of the substances involved.
The combustion reaction of starch can be represented by the following balanced chemical equation.
||\color{#FA7921}{\text{C}_6\text{H}_{10}\text{O}_5}\ +\ \color{#3A9A38}{6}\ \color{#FA7921}{\text{O}_2}\rightarrow\color{#3A9A38}{6}\ \color{#333FB1}{\text{CO}_2}\ +\ \color{#3A9A38}{5}\ \color{#333FB1}{\text{H}_2\text{O}}||
Note: When the stoichiometric coefficient of a substance is |1,| it is not written in the equation.
According to this equation, |\color{#3A9A38}{1}| molecule of starch |(\color{#FA7921}{\text{C}_6\text{H}_{10}\text{O}_5})| reacts with |\color{#3A9A38}{6}| molecules of oxygen |(\color{#FA7921}{\text{O}_2})| to produce |\color{#3A9A38}{6}| molecules of carbon dioxide |(\color{#333FB1}{\text{CO}_2})| and |\color{#3A9A38}{5}| molecules of water |(\color{#333FB1}{\text{H}_2\text{O}}).|
A chemical reaction can fit into several types of reactions at once. For example, cellular respiration is both an oxidation and a decomposition reaction.
A synthesis reaction is a chemical reaction where two or more reactants interact to form a new product.
A synthesis reaction can be recognized by analyzing the number of different reactants and products, as well as the complexity of the substances involved.
It is possible to recognize a synthesis reaction by the following clues:
There are more reactants than products.
The reactants are simpler substances than the product(s).
During the following chemical reaction, an ethylene molecule |(\text{C}_2\text{H}_4)| reacts with a hydrogen |(\text{H}_2)| molecule to form an ethane molecule |(\text{C}_2\text{H}_6).|
This is a synthesis reaction because two reactants, ethylene |(\text{C}_2\text{H}_4)| and hydrogen |(\text{H}_2),| react to form one product, ethane |(\text{C}_2\text{H}_6).| There are therefore more reactants than products. Furthermore, the two reactants are simpler molecules than the product: ethylene |(\text{C}_2\text{H}_4)| and hydrogen |(\text{H}_2)| each contain fewer atoms than ethane |(\text{C}_2\text{H}_6).|

Ethane synthesis
Photosynthesis is a synthesis reaction where carbon dioxide |(\text{CO}_2)| reacts in the presence of solar energy and water |(\text{H}_2\text{O})| to produce glucose |(\text{C}_6\text{H}_{12}\text{O}_6)| and oxygen gas |(\text{O}_2).|
It is recognized as a synthesis reaction because the glucose molecule produced |(\text{C}_6\text{H}_{12}\text{O}_6)| is more complex than the two reactants involved (|\text{CO}_2| and |\text{H}_2\text{O}|).
In plants, this chemical reaction takes place in the chloroplasts.

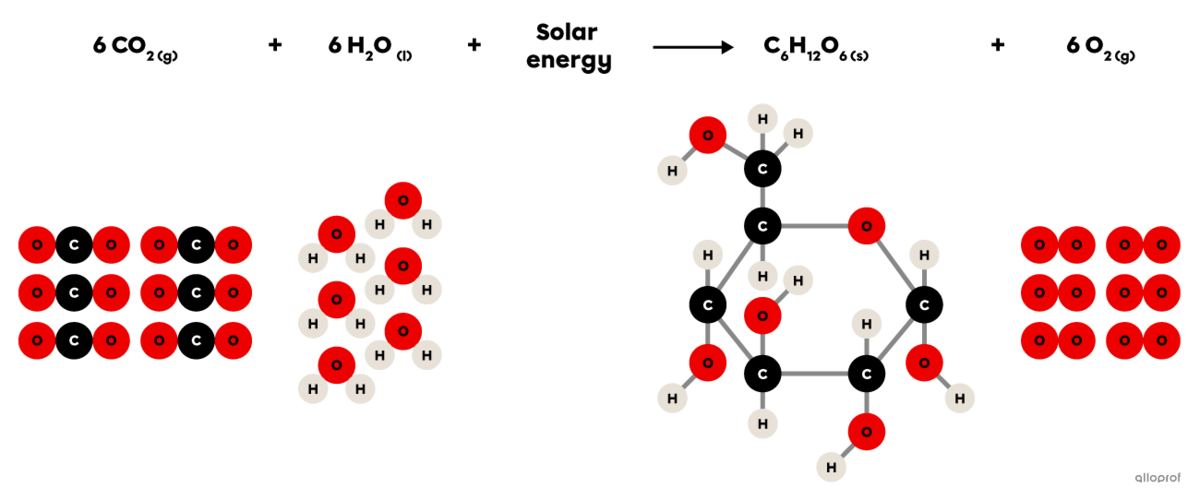
The balanced chemical equation of photosynthesis
A decomposition reaction is a type of chemical reaction where the atoms of a reactant dissociate and rearrange to form several products.
A decomposition reaction can be recognized by analyzing the number of different reactants and products, as well as the complexity of the substances involved.
It is possible to recognize a decomposition reaction by the following clues:
There are more products than reactants.
The product(s) formed are simpler substances than the reactant(s).
In the following reaction, hydrogen peroxide |(\text{H}_2\text{O}_2)| decomposes to form hydrogen gas |(\text{H}_2)| and oxygen gas |(\text{O}_2).|
It is recognized as a decomposition reaction because only one reactant, hydrogen peroxide |(\text{H}_2\text{O}_2),| is decomposed to form two products, hydrogen gas |(\text{H}_2)| and oxygen gas |(\text{O}_2).|
In addition, hydrogen peroxide |(\text{H}_2\text{O}_2)| is a more complex molecule than either of the two products: it contains more atoms than either hydrogen gas |(\text{H}_2)| or oxygen gas |(\text{O}_2).|
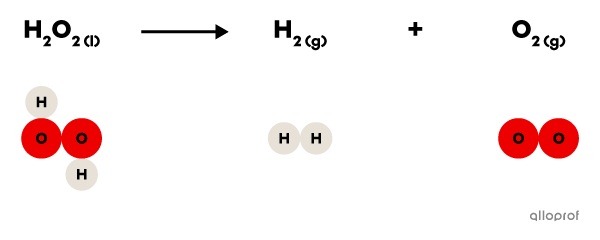
Decomposition of hydrogen peroxide
During chemical digestion, decomposition reactions allow nutrients, which are complex molecules, to be broken down into simpler molecules that can be absorbed by the body.
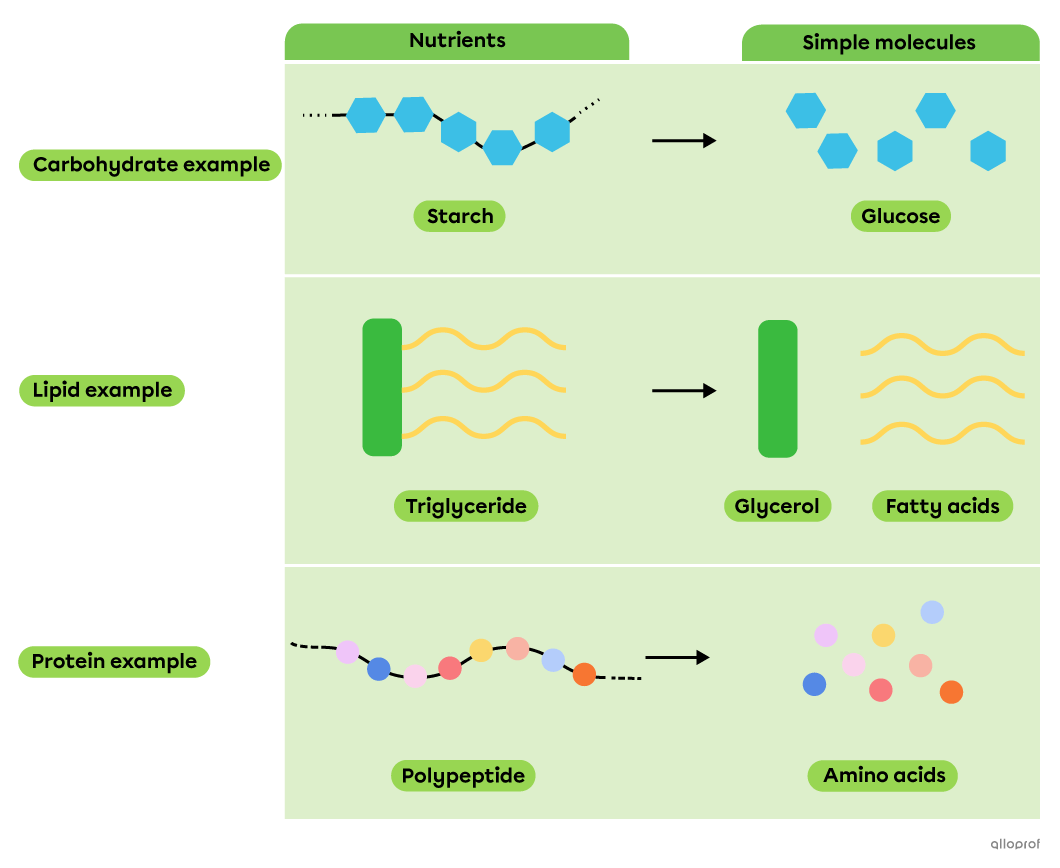
Decomposition of nutrients into simple molecules
A precipitation reaction is a chemical reaction that occurs when two substances in a solution interact to produce an insoluble or slightly soluble solid called a precipitate.
The chemical equation of a precipitation reaction can be recognized by analyzing the physical state of the substances involved in the reaction.
It is possible to recognize a precipitation reaction by the following clues:
One of the products is in a solid state.
The other substances involved are in an aqueous solution.
In a solution, sodium chloride |(\text{NaCl})| dissociates into |\text{Na}^+| and |\text{Cl}^-| ions, while silver nitrate |(\text{AgNO}_3)| dissociates into |\text{Ag}^+| and |\text{NO}_3^-| ions.
When a solution of sodium chloride |(\text{NaCl})| is mixed with a solution of silver nitrate |(\text{AgNO}_3),| the |\text{Ag}^+| and |\text{Cl}^-| ions combine to form silver chloride |(\text{AgCl}).| It is a slightly soluble or insoluble ionic compound that settles to form a whitish precipitate.
This is recognized as a precipitation reaction because the chemical equation indicates that one product, silver chloride |(\text{AgCl}),| is in a solid state and the other substances involved are in an aqueous solution.
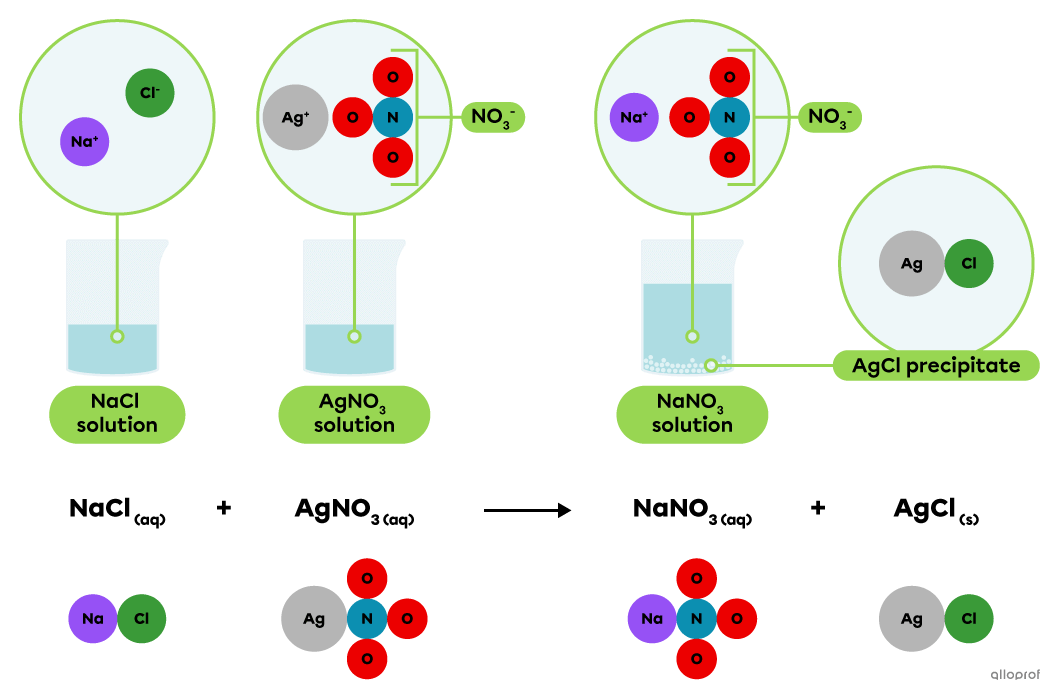
Precipitation of silver chloride
An oxidation reaction is a chemical reaction in which an element undergoes a loss of electrons. This loss of electrons is due to the presence of oxygen, or another substance with similar chemical properties, among the reactants.
An oxidation reaction can be identified by the presence of an oxidizing agent among the reactants. Oxygen |(\text{O}_2),| ozone |(\text{O}_3)| and chlorine |(\text{Cl})| are oxidizing agents often seen in oxidation reactions.
In the following reaction, iron |\text{Fe}| reacts with oxygen gas |(\text{O}_2)| to form iron oxide |(\text{Fe}_2\text{O}_3),| commonly known as rust.
This is an oxidation reaction because an oxidizing agent, oxygen |(\text{O}_2),| is one of the reactants involved in the reaction.

An oxidized nail
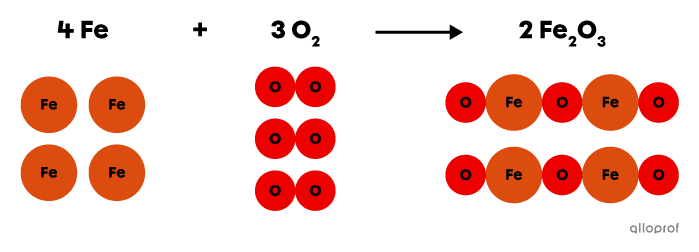
Oxidation of iron
An oxidation reaction is a chemical reaction where an element undergoes a loss of electrons. Of course, if one element gives electrons in a reaction, another element receives them. When an element receives electrons, it is a reduction reaction.
Since every oxidation reaction is accompanied by a reduction reaction, the combination of these inseparable chemical reactions is called an oxidation-reduction reaction, or a redox reaction.
A combustion reaction is a chemical reaction where an oxidation reaction occurs and energy is released.
In the following combustion reaction, propane |(\text{C}_3\text{H}_8)| reacts with oxygen gas |(\text{O}_2)| to form carbon dioxide |(\text{CO}_2)| and water |(\text{H}_2\text{O}).| In addition, the combustion of propane releases thermal energy that is commonly used for cooking or for heating buildings.

Combustion of propane
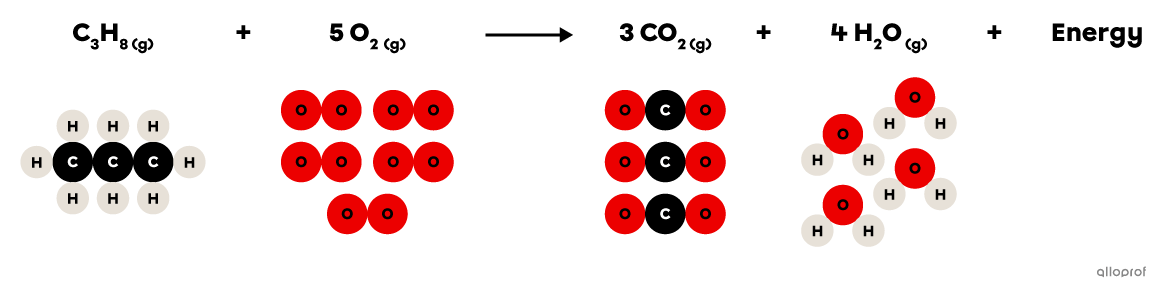
Combustion of propane
Cellular respiration is a chemical reaction that takes place in animal and plant cells.
It is an oxidation reaction where glucose |(\text{C}_6\text{H}_12\text{O}_6)| reacts with oxygen |(\text{O}_2)| to form carbon dioxide |(\text{CO}_2)| and water vapour |(\text{H}_2\text{O}).| In addition, cellular respiration produces the energy essential for the proper functioning of cells.
Since it involves an oxidation reaction and the release of energy, it can also be said that cellular respiration is a combustion reaction.
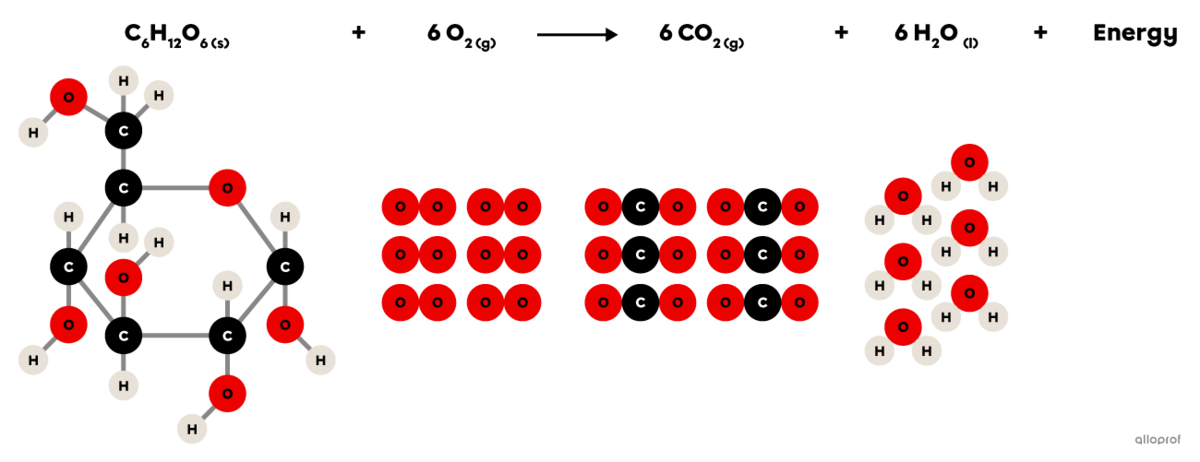
Cellular respiration
An acid-base neutralization reaction is a chemical reaction where an acid reacts with a base to produce a salt and water.
||\text{Acid}_{\text{(aq)}}\ +\ \text{Base}_{\text{(aq)}}\rightarrow\text{Salt}_{\text{(aq)}}\ +\ \text{Water}_{\text{(l)}}||
An aqueous solution of sodium hydroxide |(\text{NaOH})| can be neutralized by a hydrochloric acid solution |(\text{HCl}).| Once in a solution, these substances release ions that combine to form a sodium chloride salt |(\text{NaCl})| and water |(\text{H}_2\text{O}).|
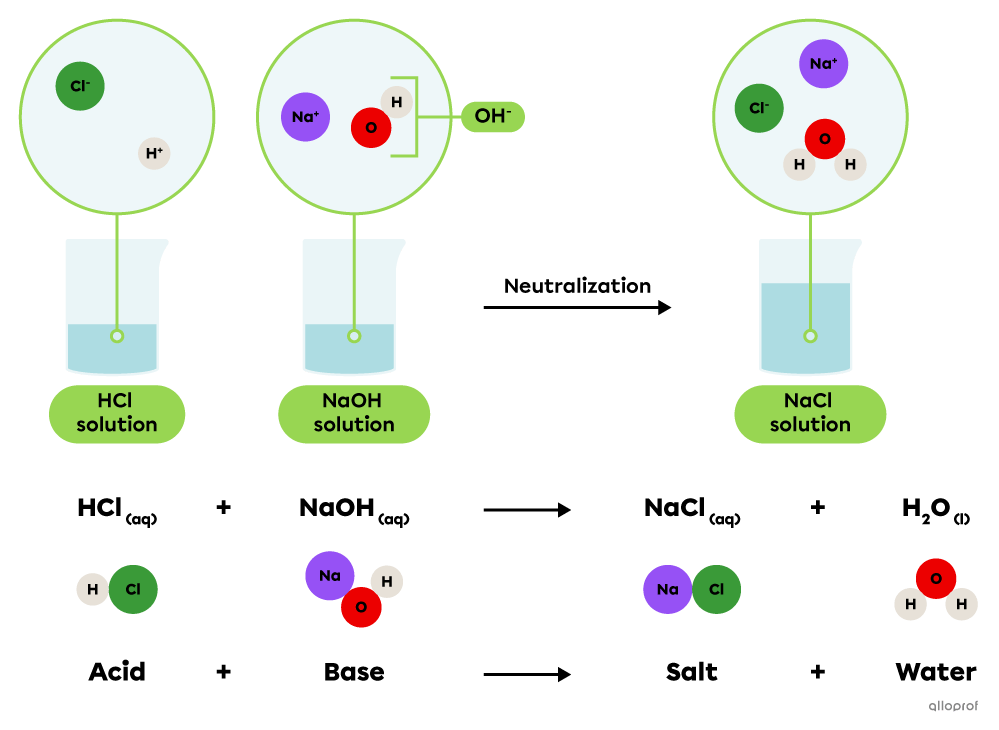
Acid-base neutralization